FAQs about Pompe treatment
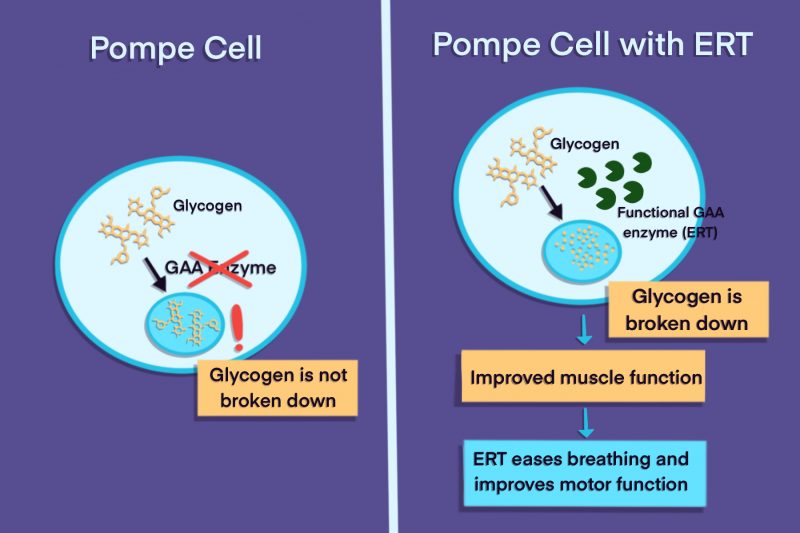
Enzyme replacement therapy, or ERT, is an approved form of treatment that addresses the root cause of Pompe disease. As the term suggests, ERT involves therapeutic administration of a version of the enzyme that is deficient in people with Pompe disease. While ERT is not a cure, it can slow the progression of the disease.
Enzyme replacement therapy (ERT) can reduce the toxic buildup of glycogen that drives Pompe symptoms, thereby slowing disease progression and easing symptom severity. In clinical trials involving babies with infantile-onset Pompe, ERT has been shown to improve survival and reduce the need for ventilation. In late-onset patients, both ERT and the combination therapy Pombiliti + Opfolda have been shown in clinical trials to improve walking ability and breathing function.
As each person may respond differently to a given treatment, there is no standard timeline for how soon a therapy starts to work. In clinical trials, ERT has been shown to improve walking ability and respiratory function in late-onset Pompe patients within about three months of starting treatment. In infantile-onset Pompe, ERT lowered levels of disease biomarkers indicative of muscle damage and glycogen excess within six months of starting treatment.
Pompe disease is caused by a deficiency of the acid alpha-glucosidase enzyme, which is needed to break down a complex sugar molecule called glycogen.
Pompe disease is caused by the toxic buildup of glycogen in the body’s cells/tissues, and the disease mainly affects muscles that rely heavily on glycogen as an energy source. The most common life-limiting complications of Pompe disease are heart disease due to damage to heart muscle, and respiratory failure due to damage of the muscles that are needed for breathing.
Related Articles

 Fact-checked by
Fact-checked by