FAQs about ERT for Pompe disease
Enzyme replacement therapy is not a cure for Pompe disease, but it can slow the disease’s progression.
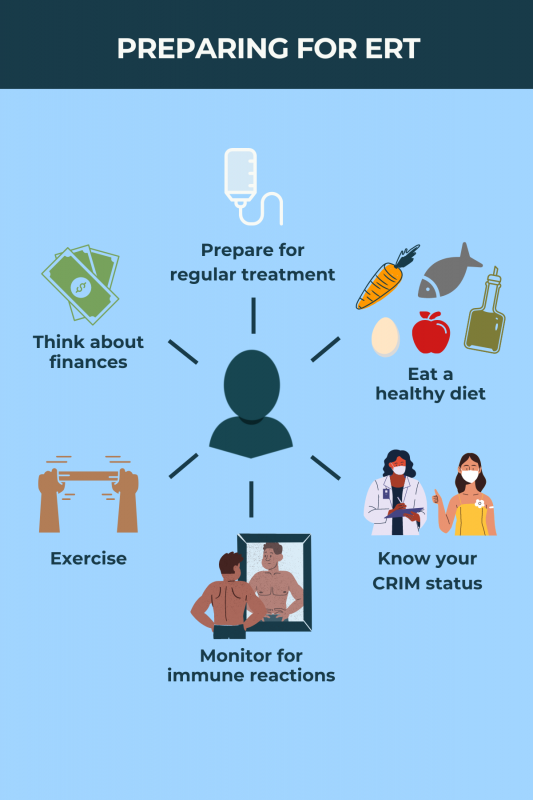
Both enzyme replacement therapies approved in the U.S. can be used in children. Lumizyme (alglucosidase alfa) is approved for Pompe patients of all ages, whereas Nexviazyme (avalglucosidase alfa) is approved for late-onset Pompe patients, ages 1 and older. An early start to treatment is generally thought to lead to better outcomes for patients.
Pompe disease is caused by genetic mutations that lead to a missing or dysfunctional acid-alpha glucosidase (GAA) enzyme, resulting in the buildup of a sugar molecule called glycogen that disrupts tissue function.
Delivered into the bloodstream, enzyme replacement therapy aims to supply a healthy version of GAA, restoring the body’s ability to break down glycogen, thereby easing Pompe symptoms.
Some patients may have allergic reactions to enzyme replacement therapy, which are rarely life-threatening. Side effects such as nausea, fever, itching, rash, or coughing may occur shortly after the treatment is infused into the bloodstream.
Other side effects reported in clinical trials include headache, fatigue, vomiting, dizziness, muscle pain, hives, shortness of breath, or increased blood pressure.
Enzyme replacement therapy is administered as an into-the-vein infusion once every two weeks. Each infusion may take four to seven hours, but this will depend on factors including dose, infusion rate, and a person’s tolerance to treatment.
 Fact-checked by
Fact-checked by